Imported Cosmetics CFDA Registration 8 Steps
The first step, clearly understand the situation of the products, imported procedure, budget and cost.
(1). Clearly know the situation of the products
As importer of the cosmetics, especially the distributor of the cosmetics, firstly needs to know the situation of the products, such as if the products have already come into the market? Whether the raw materials of the products conform to Chinese laws and regulations or not? If the products have been approved in ? What the kind of the cosmetics? Special cosmetics or normal cosmetics?
(2). Know the imported process, period and cost
Know the import process of the cosmetics. Mainly two steps, first is the CFDA registration, after the company receives the CFDA registration certificate, then the company can have the customs declaration.
Registration period of one of the most concerned questions. According to Tianjianhuacheng’ experience, imported normal cosmetics can usually finish the registration in 3-6 months, and depends on the different functions of the products, and special cosmetics can usually finish the CFDA registration in 6-12 months.
Besides, the cost of the CFDA registration is one of the most important questions that company need to take into consideration. And the detailed cost can see from our website, http://www.zhuceabc.cn/Cosmereg. Generally speaking, the total registration fee of normal cosmetic is about 10 thousand RMB, and the total fee of the special cosmetic is about 10-40 thousand RMB. (Main costs of the registration are testing fee, notary fee and translation fee.
Step 2 Signed the agency contract, verify the documents that foreign company can provide
As a distributor, it should signed the agency contract with the foreign manufacture company, the valid date of the CFDA registration is four years, so the valid date of the agency contract is better more than four years.
When Chinese distributor signed the contract with foreign companies, first needs to know whether the foreign company can provide related technique documents (such as complete formula, manufacture process, quality standards) and confirmation certificate (such as free sales certificate), because for the protection of patents, some companies don’t want to provide some of the key technologies.
In addition, somecountries do not recommend having animal testing, so before products have CFDA registration, company needs to know about this.
To know more about the documents needed for CFDA registration and its related requirements, please visit our website, http://www.zhuceabc.cn/Cosmereg/, or send us email to get more information.
Step 3 Choose the way of registration, apply by self or ask for an agency
Because now the cosmetics registration becomes more professional and difficult, and it is hard for a new established company to build a special department for cosmetics registration, so it is important for a company to choose a professional agency, http://www.zhuceabc.cn/gs, please visit our website to find how to choose a professional agency.
Step 4 Filing of the power of attorney, start the declaration
Power of attorney filing is important in the cosmetics registration. After the filing these documents, then company can have sample testing, submit the documents to CFDA for evaluation. The power of attorney is the contract that applicant authorize one other company or agency to take responsible for the registration project in China, and this power of attorney should be signed by both parties and notarized by notary public. If the documents use foreign languages, it should be translated into Chinese, and notarize the translation in. If the applicant wants to have the template of the authorization letter or the notary letter, please contract with Tianjianhuacheng.
Step 5 Sample testing
After the filing of the documents, then applicant can have the sample testing. According to the different functions and ingredients of the product, generally the testing takes about 20 days to 6 months. To find more about the details of the process, period and the fee of the testing, please visit out website, http://www.zhuceabc.cn
Step 6 Submit the documents to CFDA
After the testing of the samples, then applicant should submit the documents to CFDA for evaluation. First CFDA needs to have form evaluation, and then CFDA will inform the applicant whether CFDA will accept the declaration or not in five days.
Step 7 Documents replenish or revise
After CFDA accept the declaration, and of the submitted documents are not complete, CFDA will inform the applicant, and then applicant needs to replenish or correct the mistakes on the documents.
Step 8 Complete the registration and get the CFDA approval certificate
It usually takes 20 days (normal cosmetics) or 110 days (special cosmetics) after CFDA accept the declaration until the applicant gets approval certificate. But because usually applicant needs to replenish the documents so it may takes more time. The valid date of the certificate is four years, after the applicant receives the certificate, then company can have the import process.



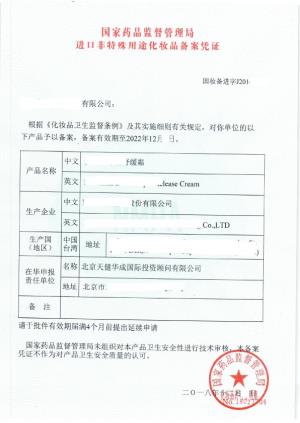
 进口化妆品批文申报注
进口化妆品批文申报注 进口化妆品注册申报及
进口化妆品注册申报及 《天健华成教你如何进
《天健华成教你如何进